

Hotline:0755-22277778
Tel:0755-22277778
Mobile:13826586185(Mr.Duan)
Fax:0755-22277776
E-mail:duanlian@xianjinyuan.cn
as everyone knows,Heating materialThere are various types, such as Ni Cr alloy type, P-NTC thermosensitive semiconductor ceramic type, SnO2-Sb metal oxide semiconductor thin film type, graphite carbon black non-metallic thick film type, and so on. This article aims to introduce a heating element made of non-metallic graphite carbon black heating materials. This type of heating element belongs to the thick film type and is composed ofresistor pasteBy coating process, an appropriate area is formed on the inorganic substrate material, and finally sintering is completed under high temperature conditions. Only the film coated under high temperature conditions has a certain resistance value. Resistance paste is made of conductive particles of non-metallic materials.
1. Thermoelectric materials
With the acceleration of global industrialization, energy and environmental issues have become the focus of attention for countries around the world. Research and development of new energy has become a global trend in energy development. At present, scientists from various countries are committed to seeking efficient and pollution-free new ways of energy conversion and utilization, in order to achieve the goal of rational and effective utilization of industrial and agricultural waste heat, waste heat from automobiles, geothermal energy, solar energy, and ocean temperature differences. Therefore, since the 1990s, the research on energy conversion materials (thermoelectric materials) has become a hot topic in materials science.
Thermoelectric materials, also known as thermoelectric materials, are functional materials that utilize the movement of internal charge carriers in solids to directly convert thermal and electrical energy into each other. They are mainly used for thermoelectric power generation and refrigeration.
The phenomenon of mutual conversion between thermal energy and electrical energy was discovered as early as the early 19th century. When there is a temperature difference at the junction of a closed circuit composed of two different conductors, current is generated in the circuit. This effect later became known as the Seebeck effect, which is the thermoelectric conversion effect that converts thermal energy into electrical energy. In 1834, J.C.A. Peltier of France discovered the inverse effect of the Seebeck effect: when current passes through a circuit composed of these two metals, heat absorption and release phenomena occur at the two junctions, which is called the Peltier effect and is the basis of thermoelectric refrigeration. In 1838, Russian physicist L. Lenz provided a correct explanation for this phenomenon through experiments: whether the joint of two conductors absorbs or releases heat depends on the direction of the current flowing through the conductor, that is, the Peltier effect is the inverse process of the Seebeck effect. In 1854, the renowned British physicist W. Thomson discovered that when there is a temperature field inside an electrified conductor, in addition to Joule heating, there is another phenomenon of heat absorption and release, known as the Thomson effect. Thomson determined the relationship between Seebeck coefficient and Peltier coefficient through thermodynamic analysis of Seebeck effect and Peltier effect, thus establishing the theoretical basis for thermoelectric phenomena.
In the 1930s, with the development of semiconductor physics, it was discovered that the Seebeck coefficient of some semiconductor materials could be higher than 100 μ V/K, leading to an increase in research on semiconductor thermoelectric materials. In 1949, the renowned semiconductor scientist A. Ioffe of the former Soviet Union discovered that the thermoelectric effect in doped semiconductors was orders of magnitude stronger than that in metals and alloys. It was hoped that based on this effect, semiconductor refrigeration could be used to manufacture household appliances. Subsequently, some materials with high thermoelectric properties such as Bi2Te3, PbTe, and SiGe were successively introduced and have been continuously used until now.
After the 1990s, with the increasing attention and support from the US government towards thermoelectric research, there has been a significant improvement in the study of thermoelectric materials. In 1993, Dresselhaus of MIT analytically proposed that based on nanotechnology and using quantum superlattices, the threshold of ZT=1 could be broken. Next, research on low dimensional materials such as quantum wells, quantum wires, quantum dots, superlattices, and thin film superlattices has surged, and many thermoelectric materials with ZT>2 have been reported successively. Some new ideas and approaches have also been proposed for the development of new thermoelectric materials, such as the new idea based on electronic crystal phononic glass (PGEC), where several materials with potential high ZT values have been discovered. Such as Skutterudite, Cathrates, Half Heusler alloy compounds, and layered cobalt oxide thermoelectric materials. All of these have laid the foundation for the further development of thermoelectric science.
When there is a potential difference between metal and semiconductor materials, current is generated, while when there is a temperature difference, heat flow is generated. Both current and heat flow are phenomena related to the movement of electrons, therefore, there exists a physical relationship between potential difference, temperature difference, current, and thermoelectric, which results in the so-called thermoelectric phenomenon. The thermoelectric effect refers to the collective term for reversible thermal effects caused by current and electrical effects caused by temperature differences. It is emphasized here that the thermoelectric effect is reversible to distinguish it from the Ohmic effect. Thermoelectric effects include Seebeck, Peltier, and Thomson effects. These three effects are not independent, they are closely connected through the Kelvin relationship. They are the physical basis for energy conversion in thermoelectric materials.
In 1823, German scientist T. Seebeck discovered a circuit composed of two different metals. If the temperatures at the two joints are different, current and electromotive force will appear in the circuit. This phenomenon is called the Seebeck effect, as shown in Figure 1-12. When two different conductors are connected in series to form a circuit, if joints 1 and 2 maintain different temperatures T1 and T2 (T1>T2), there will be a potential difference between the open circuit positions y and z of conductor b, with a value of:
Vyz=αab(T1-T2)=αabΔT
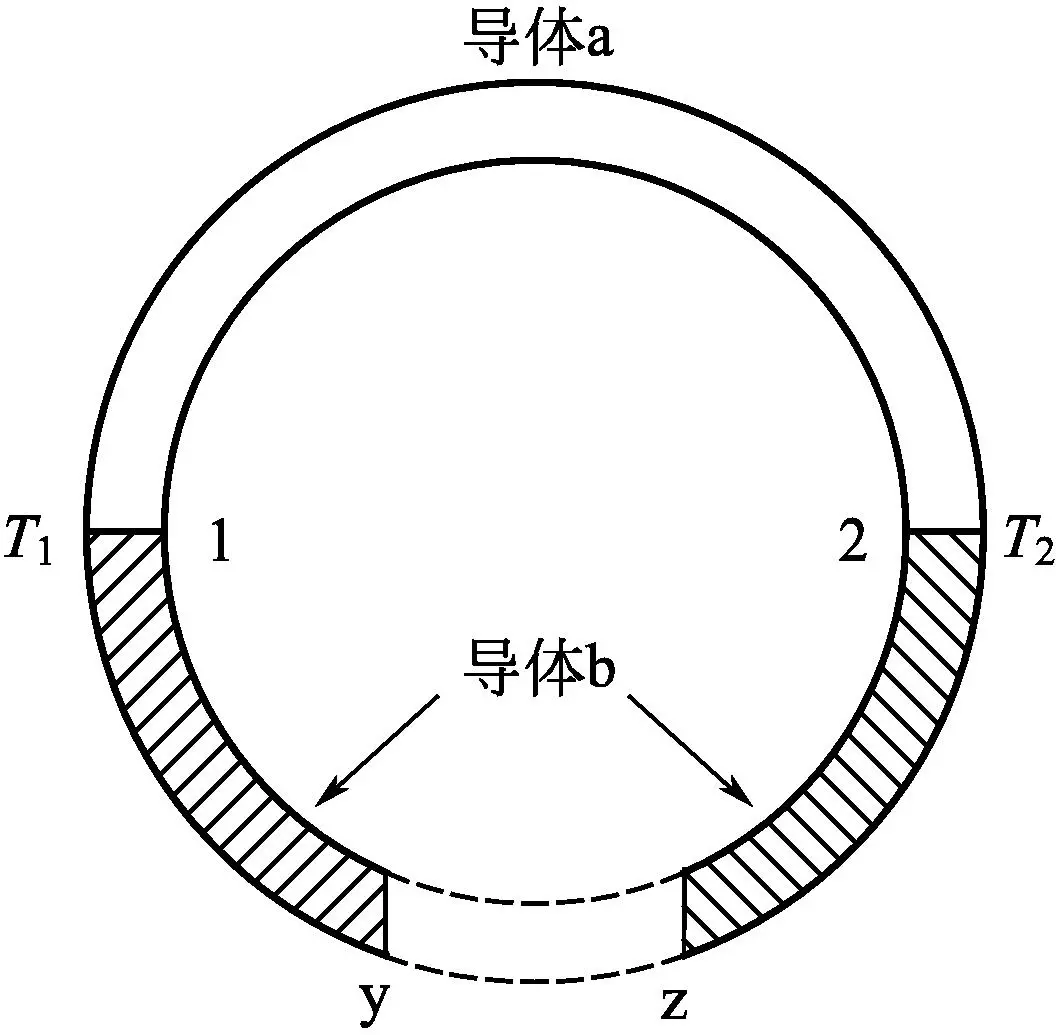
Figure 1-12 Schematic diagram of thermoelectric effect
In the equation, Δ T is not very large, and this relationship is linear, where α ab is a constant, and α ab is defined as the relative Seebeck coefficient between two variants, that is:
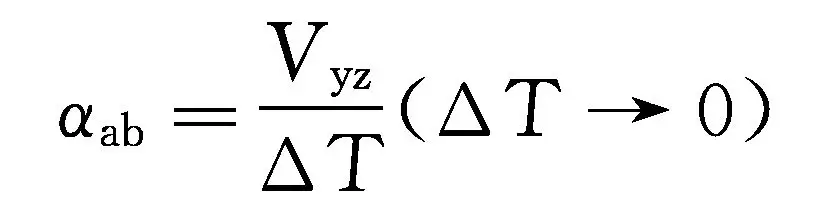
The unit of Seebeck coefficient is V/K, and due to its small value, the commonly used unit is μ V/K. The alpha value of general metal materials is 0-10 μ V/K, while the alpha value of semiconductor materials is larger, generally above 100 μ V/K. Therefore, semiconductor materials are more valuable in thermoelectric applications. There are two ways of conduction in semiconductor materials: hole conduction and electron conduction. According to the characteristics of the material, the Seebeck coefficient can also be positive or negative, corresponding to P-type and N-type thermoelectric materials, respectively. Metal materials mainly rely on electrons for transmission, and their Seebeck coefficient is negative.
In 1834, French physicist C.A. Peltier discovered that when current flows through nodes of two different materials (conductors or semiconductors), one node will release heat while the other node will absorb heat. This phenomenon is called the Peltier effect, as shown in Figure 1-12. If a voltage is applied across y and z in Figure 1-12, a current I will flow through the circuit formed by conductor a and conductor b. Heat absorption and release phenomena will occur at the joint of the two conductors, respectively. Let the heat absorption (or release) rate at the joint be q, and q is proportional to the current I, that is:
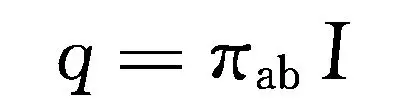
In the equation, π ab is a proportionality constant defined as the Peltier coefficient, i.e.:
The Peltier coefficient represents the amount of heat absorbed (or released) by a unit of current at a junction per unit time, measured in W/A, and can also be expressed in units of voltage V. The size of π ab is related to the contact temperature and the material composition of the thermocouple. The Peltier effect is the inverse of the Seebeck effect, which is caused by the different concentrations of charge carriers and Fermi levels in the materials on both sides of the junction. When current passes through the junction, energy exchange with the environment is necessary to maintain energy and charge conservation.
When there is both a temperature gradient and a current passing through a circuit, in addition to generating Joule heat related to resistance, the circuit also needs to absorb and release heat. This effect is called Thomson effect, and the heat generated is Thomson heat. The amount of heat absorbed or released per unit time and volume is proportional to the current density and temperature gradient, that is:
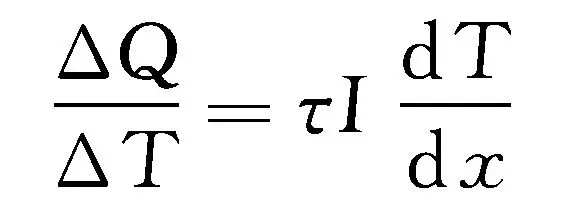
In the formula, τ is the Thomson coefficient; X is the spatial coordinate.
The positive and negative rules of the expression are the same as the Peltier effect. When the current flows towards the hot end, dT>0,τ>0, If Δ Q>0, it will absorb heat, otherwise it will release heat.

Advanced Institute (Shenzhen) Technology Co., Ltd, © two thousand and twenty-onewww.avanzado.cn. All rights reservedGuangdong ICP No. 2021051947-1 © two thousand and twenty-onewww.xianjinyuan.cn. All rights reservedGuangdong ICP No. 2021051947-2